Abstract
Background The introduction of dexamethasone for the treatment of pediatric acute lymphoblastic leukemia (ALL) significantly contributed to the current overall survival rate of more than 90% in high-income countries. The downside however, is that dexamethasone can induce serious side effects, of which neurobehavioral side effects are experienced as detrimental with respect to health-related quality of life (HRQoL) by both patients and parents. Our previous study suggests that children who suffer most from neurobehavioral side effects might benefit from physiological hydrocortisone addition to dexamethasone treatment. We aimed to validate this finding in a prospective large cohort of pediatric ALL patients.
Methods Our phase 3, double-blind, randomized controlled trial with crossover design included ALL patients (3-18 years) during medium risk maintenance therapy in a national tertiary hospital between May 17, 2018 and August 5, 2020. A baseline measurement before and after one five-day dexamethasone course was performed to identify patients with clinically relevant dexamethasone-induced neurobehavioral problems, defined as a rise of ≥5 points on the Strengths and Difficulties Questionnaire (SDQ) Total Difficulties score. These patients were subsequently randomized to receive an intervention during the following four five-day dexamethasone courses. The intervention consisted of two courses hydrocortisone (physiological dose of 10mg/m2/day in a circadian rhythm), followed by two courses placebo, or vice versa. Neurobehavioral problems were assessed before and after each course using the parent-reported SDQ as primary endpoint. Secondary endpoints were sleep problems, HRQoL, hunger feeling, and parental stress, measured with separate questionnaires and actigraphy. A generalized mixed model was estimated to study the intervention effect.
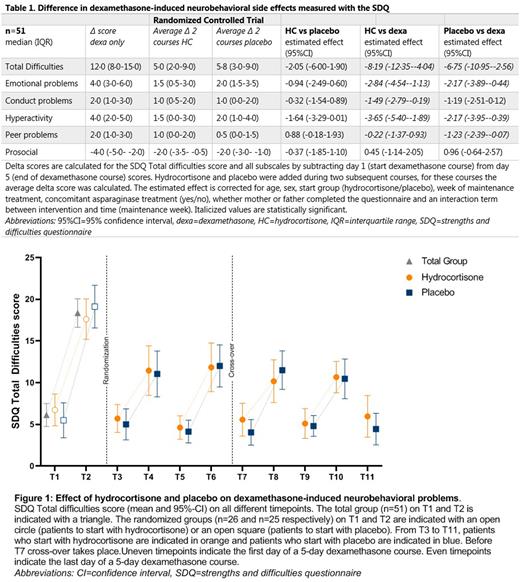
Findings Of 79 included patients, 52 (66%) experienced clinically relevant dexamethasone-induced neurobehavioral problems. Both hydrocortisone and placebo significantly and relevantly decreased dexamethasone-induced neurobehavioral problems measured with the SDQ (estimated effect hydrocortisone: -8.19 (95%-confidence interval (CI) -12.35 to -4.04), estimated effect placebo: -6.75 (95%-CI -10.95 to -2.56) (table 1 and figure 1). This indicates that, when adding hydrocortisone or placebo to dexamethasone treatment, the SDQ Total Difficulties score is estimated to be 8.19 or 6.75 points lower than without the addition of hydrocortisone or placebo. Since one SD of SDQ norm values is between 4 and 6 points, the decrease on the SDQ after hydrocortisone or placebo addition is more than 1 SD, and therefore clinically significant. The strength of the effect was similar in both interventions. There was no benefit from hydrocortisone or placebo in reducing sleep problems, hunger, parental stress or improving HRQoL.
Interpretation Clinically relevant dexamethasone-induced neurobehavioral problems decreased equally and significantly after both hydrocortisone and placebo addition. Using the placebo effect in clinical practice could be a powerful and innovative way to diminish neurobehavioral problems in children who experience clinically relevant dexamethasone-induced side effects. Moreover, the high proportion of patients who experience clinically relevant dexamethasone-induced neurobehavioral problems might be caused by a nocebo effect of dexamethasone.
By offering both interventions (hydrocortisone and open-label placebo) in clinical practice and by diminishing the nocebo effect of dexamethasone, we hope to improve dexamethasone-induced side effects in those patients who suffer most.
Disclosures
van den Akker:Rhythm Pharmaceuticals Inc: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal